Addition of Enological Tannins at the Beginning of Pinot Noir Maceration:
Impact on Color StabilizationDavid Llodrá, Research & Development Director
Oak Solutions Group
INTRODUCTION
Some vineyards planted with red wine varietals have a history of producing grapes that lack in color. Among the varietals affected, this is a particularly common issue for certain clones of Pinot noir. Trials suggest oak tannin is able to help facilitate color fixation in these situations by helping to promote bonding of anthocyanins and condensed tannins.
Rodney Strong Vineyards, Enotrex and Oak Solutions Group joined forces during the 2010 harvest to study the use of trū/tan enological tannins and another tannin brand to fix and stabilize color in a challenging lot of Pinot noir and compare this with the traditional use of French oak powder. The objective of the experiment was to identify the most effective treatment to benefit color fixation and stability during a commercial size trial.
METHODOLOGY
Grapes
The team selected a vineyard planted with the Pommard Clone of Pinot noir. This particular lot used by Rodney Strong and grown in the River West vineyard has a history of exhibiting poor color and therefore typically yields light-colored wines.
Oak Treatments
Two enological tannins were selected for evaluation:
trū/tan: a blend of gallo and ellagic oak tannin (hydrolysable tannins1). Oak is toasted, and then tannin is extracted and blended into three formulas. This experiment focused on the f² fermentation formula, which contains the highest tannin concentration of all the formulas. The tannin is sold as a soluble powder that dissolves completely in wine.
BrandX: consists of chestnut (hydrolysable tannin1) and quebracho (condensed tannin2). This brand is sold as a tannin product to fix and stabilize color and is widely used in the wine industry.
An additional oak treatment was also included:
ēvOAK untoasted French Oak Powder: consists of cooperage-quality oak that has been ground into a powder form. While enological tannins are usually preferred to fix color due to higher tannin content, French oak powder is also commonly used since it offers the fastest extraction of any oak alternative product. Untoasted French oak powder was selected to maximize the tannin available for extraction (since tannin is sensitive to heat).
1 Hydrolysable tannins (oak tannin) bind with proteins by hydrophobic interactions.
2 Condensed tannins (present in grapes and quebracho) bind with proteins through hydrogen bonding.
Conducting the Experiment
The grapes were distributed as evenly as possible across all the tanks to end up with similar fruit quality in each treatment. Each tank had a capacity 3,550 Gallons (13,438.21 Liters), and due to the tall and narrow shape, a drain and return had to be applied early in the fermentation to achieve enough extraction from the grapes. This was followed by a whole volume pump over three times a day until the end of the fermentation. Samples were pulled daily after the morning pump over and they were analyzed for all the parameters described later during the alcoholic fermentation. After pressing each lot after alcoholic fermentation, the wines were put in barrels for malolactic fermentation and samples were pulled every 2 weeks. Analyses were conducted throughout the process, from the reception of the fruit until after completion of malolactic fermentation (150 days).
BrandX was used to compare with trū/tan f 2 at the dose rates recommended by the producers. trū/tan f 2 contains the largest percentage of total tannin, and therefore a lower dose rate was used when compared to the dose rate for BrandX, as seen in the protocol below. An equivalent dose rate for French oak powder was used; presuming that approximately 4 percent by weight will be extracted as tannin, powder applied at 5g/L achieves a similar dose rate to tannin applied at 20 g/hL.1
- Control (Pinot noir without tannin or oak additions)
- ēvOAK Untoasted FO Powder at 5g/L (equivalent to 20g/hL)
- trū/tan f 2 (oak tannin) at 10g/hL
- trū/tan f 2 (oak tannin) at 20g/hL
- BrandX (Chestnut and quebracho tannins) at 20 g/hL
- BrandX (Chestnut and quebracho tannins) at 30 g/hL
In the following sections there is a brief explanation of the type of analyses performed and the interpretation of some parameters using a percentage change to compensate for the variability among the tanks used in the experiment.
WHAT WAS MEASURED
During the trial, quantitative measurements were generated of selected classes of red wine phenolic compounds that have been shown to have a close and demonstrable link to the wine’s appearance and mouthfeel; more specifically, to the perception of its color, astringency, and overall tactile sensation. These classes of phenolic compounds included anthocyanins, proteinprecipitable phenolics, and iron-reactive phenolics. Each of these classes is further discussed in the paragraphs that follow.
Anthocyanins
Anthocyanins form the basis for the wine’s color perception and contribute key ingredients to its underlying structure. These anthocyanins are present in quite a number of natural glycosidic forms, the most prominent of which being the flavylium cation form, which absorbs visible light and therefore is responsible for the wine’s red color, and the chemically-complexed form, which is the product of bindings between anthocyanin and tannin molecules. As such, anthocyanins in the flavylium cation form are referred to as free anthocyanins, while the chemically-complexed form is denoted as bound anthocyanins©³. Anthocyanins linked to tannins form stable color, and contribute significantly to the wine’s textural qualities. Consequently, three manifestations of the content of anthocyanins in red wine were measured. Namely, the levels of free anthocyanins, bound anthocyanins©, and total anthocyanins, all reported in parts-per-million malvidin equivalents (ppm ME).
Protein-Precipitable Phenolics
The condensed tannins extracted from grapes during fermentation are polymeric phenolic compounds that have the ability to bind with the salivary proteins that provide lubrication in the mouth, and ultimately precipitate from solution. This connection is believed to be one of the leading factors in the perception of the wine’s astringency, and as such, it has been the focus of a considerable number of recent investigations. However, component-based measurements of a wine’s tannin content have been difficult to date. This is primarily due to the significant number of subunits comprising the individual molecules, the polymeric nature of the tannin complex, and the morphology of the ensuing anthocyanins-tannins interactions. Fortunately, for winemaking decisions in production operations, at least in today’s environment, it is not necessary to know the concentrations of the individual tannin species present. Instead, measurement of the total amount of tannins, or more specifically, the amount of tannins that precipitate when exposed to salivary proteins, provides a more representative indicator of the tannin content in wine or juice.
³ Bound anthocyanin© is copyrighted by Enotrex. Permission to use this name was granted to Oak Solutions Group for the purpose of this document as part of the collaboration.
Iron-Reactive Phenolics
The incredible diversity of the structures of phenolic compounds in red wine, as well as their associated interactions that give way to their sensory impact, has presented a formidable challenge in terms of achieving measurements that focus on single entities or individual family components. On the other hand, measurement of the total amount of phenolic compounds in red wine is considered to have been rather successful in providing an overall indicator of the quality of the originating fruit, the extent of extraction that took place during maceration, and the influence of exogenous enological tannins. The specific method used in this study for measuring the wine’s total phenolics is that of Herbertson, et al, 2002. It is based on a ferric chloride reagent and, because of its particular specificity, the resulting measurement is referred to as total iron-reactive phenolics.
Color Analysis
To address the perception of color, red wine color was measured in accordance with the widely-used CIE L*a*b* international standard developed and supported by the Commission Internationale de l’Eclairage (CIE) in Vienna, Austria. This standard uses the visible region of the light spectrum to measure the absorbance of light by the wine, and represents its color with three parameters: L* (“degree of lightness”), a* (red or green content), and b* (yellow or blue content). The lightness coordinate L* ranges from zero (dark, black) to one hundred (light), while the a* coordinate is greater than zero (red, no green component) and the b* coordinate may be positive (yellow only) or negative (blue only). The difference in hue between two samples, is reflected in their a* and b* coordinate values.
HOW THEY WERE MEASURED
The time evolution of phenolic compounds present in each trial was measured using an extended version of the Harbertson & Adams Assay, Harbertson et al, 2002. This assay relies on four fundamental characteristics of the behavior of wine phenolic compounds when they come in contact with protein, free sulfur dioxide, and ferric chloride. Namely, these underlying principles include: the pH behavior of anthocyanins in solution in the acidic region, the ability of a solution of potassium metabisulfite to bleach anthocyanins, the capability of proteins to form complexes with tannins and precipitate from solution, and the reactivity of ferric chloride with phenolic compounds that possess vicinal dihydroxyl groups. The families of measured phenolic compounds consisted of total anthocyanins, free anthocyanins, bound anthocyanins©, protein-precipitable tannins, and iron-reactive phenolics.
The CIE L*a*b* color coordinates were determined by first scanning the absorbance of light by the wine samples from 340 nm to 780 nm using a visible region spectrophotometer, and then using this spectrum to calculate the corresponding tri-stimulus color parameters. In accordance with the CIE L*a*b* standard, the CIE Illuminant D65 and the CIE (1964) 10-degree Color Matching Functions were used in the underlying calculations.
RESULTS
Figure 1. Formation of Bound Anthocyanins© — Impact on Color Stabilization
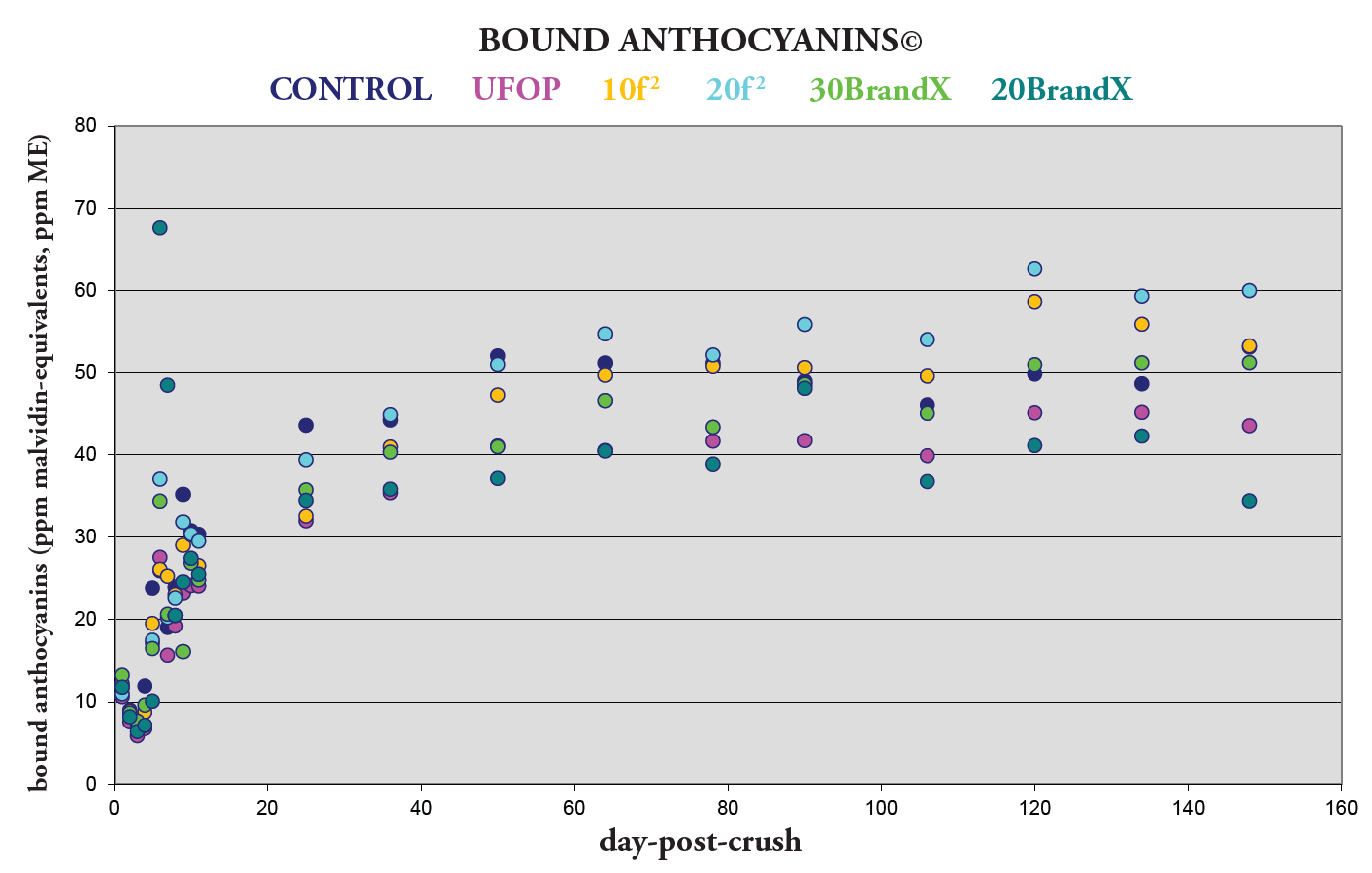
Figure 2. Percentage Change of Bound Anthocyanins©
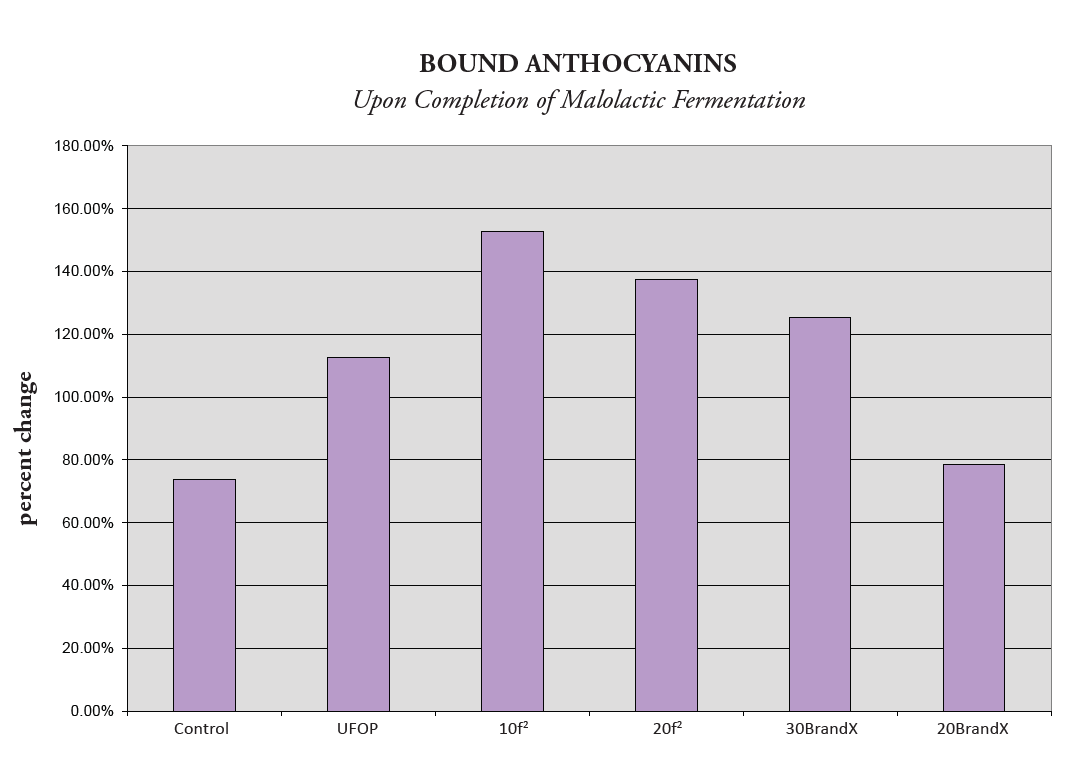
However, the best way to evaluate this transformation is by analyzing Figure 2. This shows the percentage change of bound anthocyanins©, and as expected from Figure 1, both of the trū/tan f 2 treatments showed a larger percentage change, even with lower dose rates than BrandX.
Figure 3. Percentage Change of Free Anthocyanins
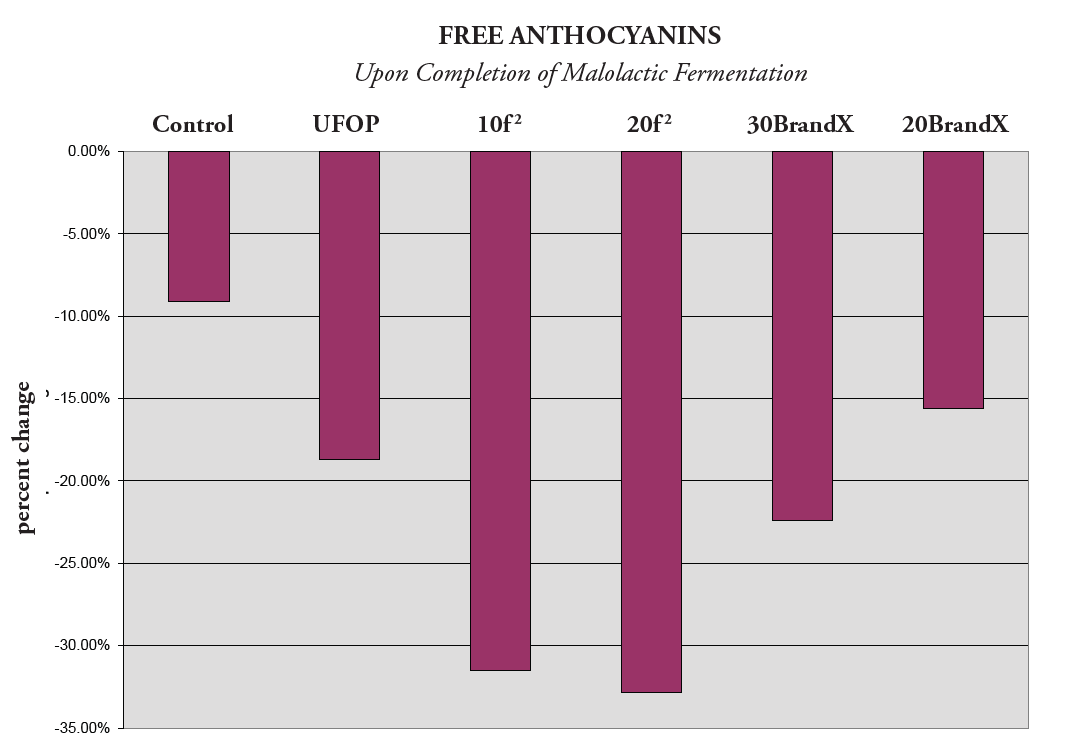
It is also helpful to evaluate the percentage change in free anthocyanins during this same period, as shown in in Figure 3. A decrease in free anthocyanins can happen for two reasons: precipitation and/or binding to condensed tannins. Therefore, one would expect to see a decrease in free anthocyanins since Figure 1 and 2 demonstrate bound anthocynanins© are being successfully formed. For this reason, the Y axis contains negative percentage values; free anthocynanins are decreasing, which serves as a positive indicator for color fixation and stability since this is correlated to an increase in bound anthocynanins in the previous figures.
The two treatments with trū/tan f 2 were found to have the largest percentage increase in bound anthocyanins© and also the largest decrease in free anthocyanins. Therefore, we can conclude that this product had the most significant impact on promoting the binding of tannin with free anthocyanin.
Figure 4. Tannin Concentration
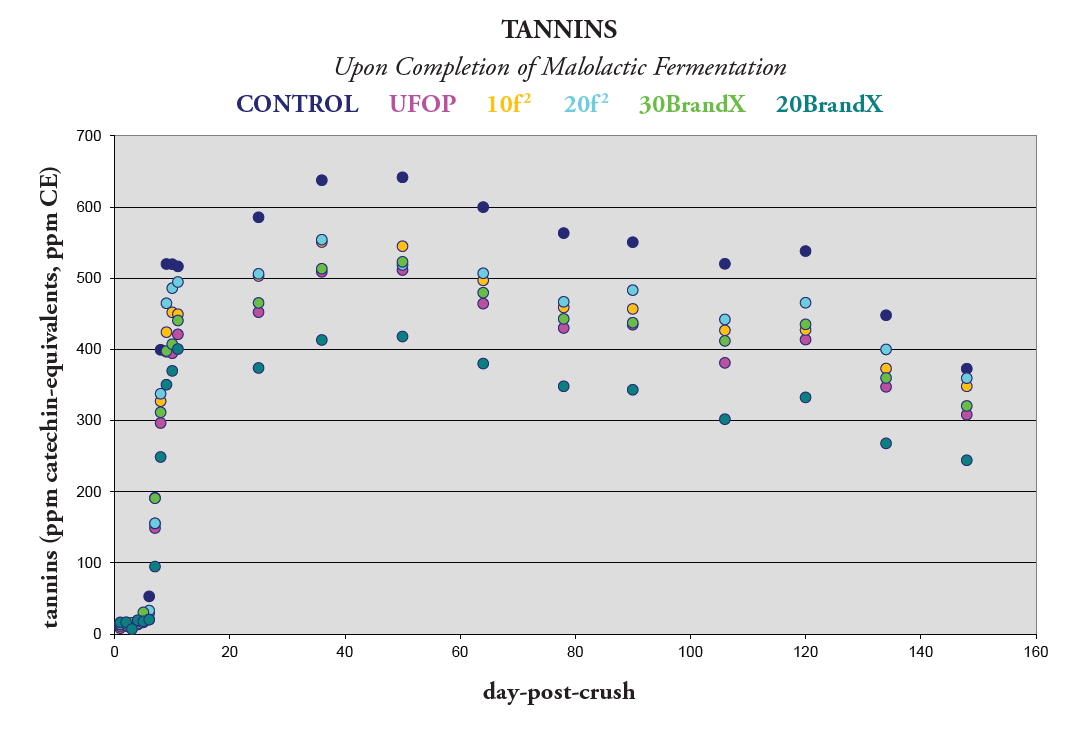
Another important measurement is the total tannin content of the wine, which allows us to evaluate if there is enough tannin to bind with free anthocyanins and fix color in red wines. In Figure 4, tannin concentration decreases after the peak is achieved during the extraction period. This follows the trend of increasing bound anthocyanins©.
BrandX is composed of both condensed and hydrolysable tannin. It is important to note that adding condensed tannin did not have a positive effect on color stability. The current understanding is that there is already a sufficient amount of condensed tannin present in the grapes, and when extracted properly, provides enough condensed tannin in solution to fix color without additional input.
Figure 5. Impact on Color Perception
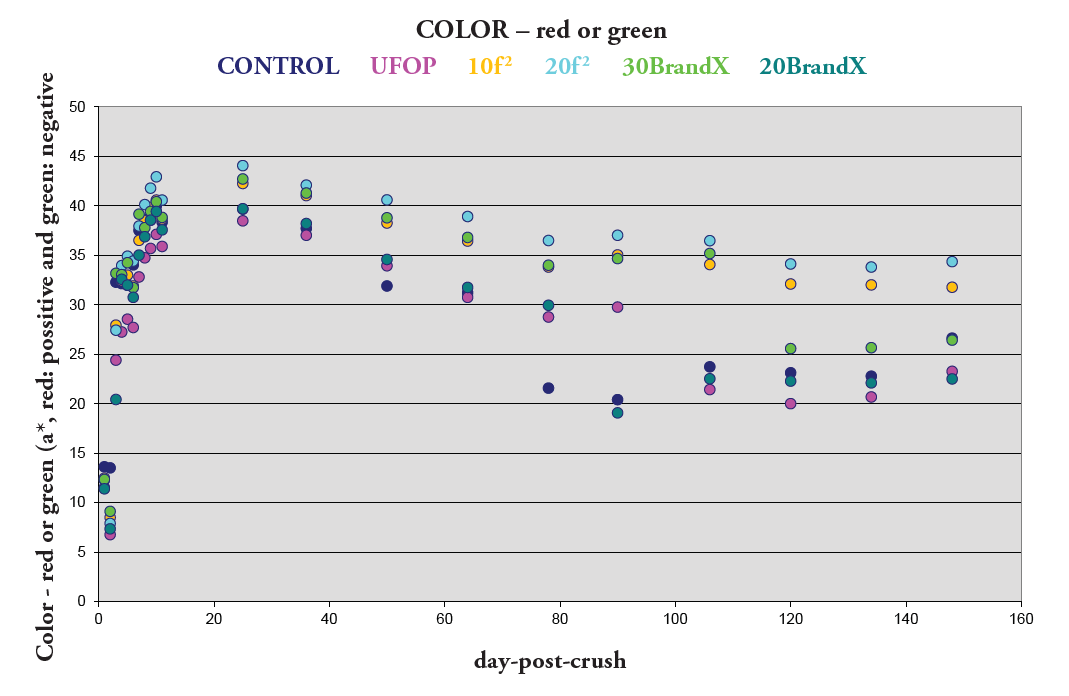
All of the previous measurements are useful, but the visual effect on wine color can be considered the most important because it is the first impression made prior to tasting and can bias the taster. In Figure 5, the results from the CIE for red color are plotted (positive values for red color and negative for green that is not present in red wine). Again, the trū/tan f 2 treatments show more red color than the rest of treatments. Because red color is more intense at the peak of anthocyanin extraction, a decrease is observed during malolactic fermentation and later during the aging period.
Figure 6. Percentage Change of Red Color
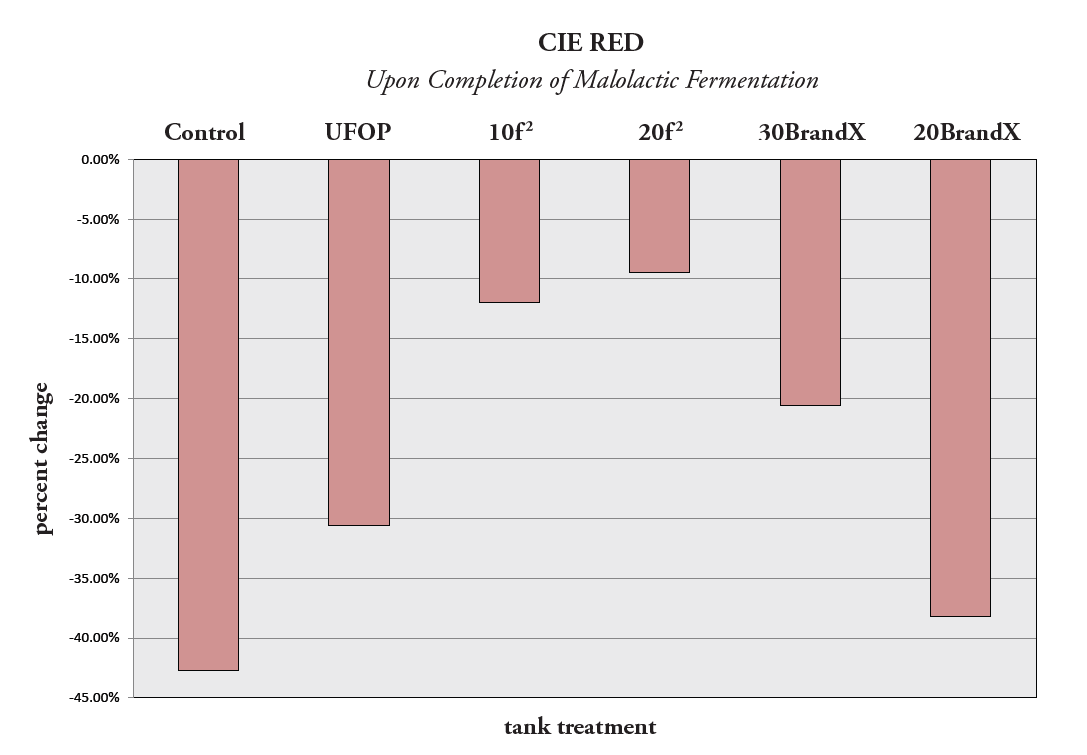
In Figure 6, the percentage change of red color upon the completion of malolactic fermentation is shown. The least amount of red color decrease (negative percentage change values) occurred in the trū/tan f 2 treatments, as expected from all the previous analysis results.
CONCLUSION
When color is a problem in the production of red wine, enological tannins are an effective tool to help fix and stabilize color. It is imperative to promote extraction of anthocyanins early in the alcoholic fermentation and there must be enough condensed tannins present to facilitate binding with the free anthocyanins. The earlier the bound anthocyanins© are formed, the higher the color intensity will be and a higher level of color stability will be achieved.
All analyses conducted on this Pinot noir Pommard Clone sourced from River West vineyard unanimously demonstrate that trū/tan f 2 tannins had the highest impact on color fixation and stabilization when compared to other commercial tannin, even at lower dose rates. The percentage changes in free anthocyanins and bound anthocyanins© and overall color analysis prove that the most significant results can be achieved using this product.
Therefore, we recommend the use of trū/tan f 2 tannins at the beginning of the alcoholic fermentation at a dose rate of 10 to 20 g/hL to promote the formation of bound anthocyanins© that fix and stabilize red color.
It is also relevant to note that untoasted French oak powder performed moderately well in this scenario and consequently continues to serve as a viable option. However, it will take longer to extract the tannins and must be applied at a high dose rate to have a significant impact. In this experiment, untoasted French oak powder was applied at 5 g/L with the goal of achieving a similar tannin impact as enological tannins. Yet it is understood that any dosage exceeding 3 g/L will impart oak aromatics, including potential woody notes. If additional oak impact is not expected or desired, enological tannins remain the ideal choice to contribute tannin without imparting oak aromatics.
ACKNOWLEDGMENTS
Oak Solutions Group would like to thank:
Greg Morthole, a winemaker at Rodney Strong Vineyards, for implementing and monitoring the experiment.
Dr. Giovanni Colantuoni of Enotrex for providing analytical services.
REFERENCES
(1) Harbertson, J. F., Kennedy, J. A., and Adams, D. O., Tannin in Skins and Seeds of Cabernet Sauvignon, Syrah, and Pinot Noir Berries During Ripening, Am. J. Enol. Vitic. 53:1 54-59 (2002).
(2) Harbertson, J. F., Picciotto, E. A., and Adams, D. O., Measurement of Polymeric Pigments in Grape Berry Extracts and Wines Using a Protein Precipitation Assay Combined with Bisulfite Bleaching, Am. J. Enol. Vitic. 54:4 301-306 (2003).
(3) Harbertson, J. F., and Spayd, S., From the ASEV 2005 Phenolics Symposium - Measuring Phenolics in the Winery, Am. J. Enol. Vitic. 57:3 280-288 (2006).
(4) Kennedy, J. A., Ferrier, J., Harbertson, J. F., and Peyrot des Gachons, C., Analysis of Tannins in Red Wine Using Multiple Methods: Correlation with Perceived Astringency, Technical Brief, Am. J. Enol. Vitic. 57:4 481-485 (2006).
(5) Commission internationale de l’Eclairage (CIE, International Commission on Illumination), CIE L*a*b Color Space, CIE Publication 15.2 (1986).
